
pH of .2 M of NH3 (weak base). More free lessons at: http://www.khanacademy.org/video?v=gDJtOIxDu78
From playlist Chemistry

Equivalence point when titrating a weak acid More free lessons at: http://www.khanacademy.org/video?v=gbpc_JBG1F0
From playlist Chemistry
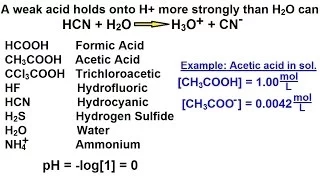
Chemistry - Acids & Bases Fundamentals (22 of 35) What Is A Weak Acid?
Visit http://ilectureonline.com for more math and science lectures! In this video I will explain "What is a weak acid?" (A "weak" acid is "strong".)
From playlist CHEMISTRY 22 ACIDS AND BASES

Chemistry - Acids & Bases (31 of 45) Comparing Acid Strengths Using % Concentrations
Visit http://ilectureonline.com for more math and science lectures! In this video I will explain the % ionized of a strong, weak, weaker, and very weak acid.
From playlist CHEMISTRY 22 ACIDS AND BASES

IB Strong and Weak Acids and bases
IB SL Chemistry lesson on strong and weak acids and bases
From playlist IB Chemistry
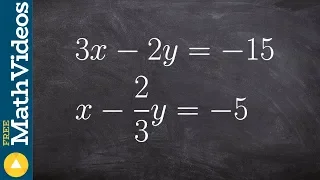
Solve a system of equation when they are the same line
👉Learn how to solve a system (of equations) by elimination. A system of equations is a set of equations which are collectively satisfied by one solution of the variables. The elimination method of solving a system of equations involves making the coefficient of one of the variables to be e
From playlist Solve a System of Equations Using Elimination | Medium

Calculating the pH of a weak acid More free lessons at: http://www.khanacademy.org/video?v=dencuBNp_Ck
From playlist Chemistry

Solve a System of Equations Using Elimination
👉Learn how to solve a system (of equations) by elimination. A system of equations is a set of equations which are collectively satisfied by one solution of the variables. The elimination method of solving a system of equations involves making the coefficient of one of the variables to be e
From playlist Solve a System of Equations Using Elimination | Hard

Eduard Feireisl: Stability issues in the theory of complete fluid systems
Find this video and other talks given by worldwide mathematicians on CIRM's Audiovisual Mathematics Library: http://library.cirm-math.fr. And discover all its functionalities: - Chapter markers and keywords to watch the parts of your choice in the video - Videos enriched with abstracts, b
From playlist Mathematical Physics

This chemistry video tutorial explains how to calculate the pH of a buffer solution using the henderson hasselbalch equation. It explains the concept, components, and function of a buffer solution. A buffer solution consist of a weak acid and its conjugate weak base counterpart. It's pu
From playlist New AP & General Chemistry Video Playlist

Solve a System of Equations with Elimination when Your Solutions are Fractions
👉Learn how to solve a system (of equations) by elimination. A system of equations is a set of equations which are collectively satisfied by one solution of the variables. The elimination method of solving a system of equations involves making the coefficient of one of the variables to be e
From playlist Solve a System of Equations Using Elimination | Hard

Lec 22 | MIT 5.111 Principles of Chemical Science, Fall 2005
Acid-Base Equilibrium (cont.) (Prof. Catherine Drennan) View the complete course: http://ocw.mit.edu/5-111F05 License: Creative Commons BY-NC-SA More information at http://ocw.mit.edu/terms More courses at http://ocw.mit.edu
From playlist MIT 5.111 Principles of Chemical Science, Fall 2005

MIT RES.TLL-004 Concept Vignettes View the complete course: http://ocw.mit.edu/RES-TLL-004F13 Instructor: George Zaidan In this video, Legos¬ are used to create possible molecular level models of a buffer. This is done to better understand how a buffer works and the components a buffer m
From playlist MIT STEM Concept Videos

Acid Base Titration Curves - pH Calculations
This chemistry video tutorial provides a basic introduction to acid base titrations. It shows you how to calculate the unknown concentration of an acid solution and how to determine the volume of base added to completely neutralize the acid and to reach the equivalence point. It also exp
From playlist New AP & General Chemistry Video Playlist

pH of Weak Acids and Bases - Percent Ionization - Ka & Kb
This chemistry video explains how to calculate the pH of a weak acid and a weak base. It explains how to calculate the percent ionization of a weak acid using the acid dissociation constant Ka. Access The Full 1 Hour 33 Minute Video on Patreon: https://www.patreon.com/MathScienceTutor/po
From playlist New AP & General Chemistry Video Playlist

IB HL Chemistry lesson on pH curves, buffers, and indicator ranges
From playlist IB Chemistry
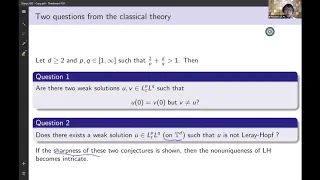
Sharp nonuniqueness for the Navier-Stokes equations - Xiaoyutao Luo
Analysis Seminar Topic: Sharp nonuniqueness for the Navier-Stokes equations Speaker: Xiaoyutao Luo Affiliation: Duke University Date: November 30, 2020 For more video please visit http://video.ias.edu
From playlist Mathematics
22. Acid-Base Equilibrium: Salt Solutions and Buffers
MIT 5.111 Principles of Chemical Science, Fall 2014 View the complete course: https://ocw.mit.edu/5-111F14 Instructor: Catherine Drennan A buffer helps to maintain a constant pH. Our blood has a natural buffering system to ensure that the pH of our blood stays within a narrow window and t
From playlist MIT 5.111 Principles of Chemical Science, Fall 2014
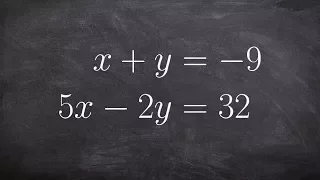
Solve a System of Equations by Using Elimination of Multiplying
👉Learn how to solve a system (of equations) by elimination. A system of equations is a set of equations which are collectively satisfied by one solution of the variables. The elimination method of solving a system of equations involves making the coefficient of one of the variables to be e
From playlist Solve a System of Equations Using Elimination | Medium
