
Specific heat capacity explained, measured and demonstrated: by fizzics.org
Specific heat capacity of a material is the quantity which describes how much energy is required to raise the temperature. The lesson demonstrates the amount of energy supplied to heat water and the specific heat capacity of the water is measured with a calculation. Notes on specific heat
From playlist Thermal energy/heat

What Is The Difference Between Specific Heat Capacity, Heat Capacity, and Molar Heat Capacity
This chemistry video tutorial explains the difference between specific heat capacity, heat capacity, and molar heat capacity. It contains a few examples and practice problems on calculating the specific heat capacity and molar heat capacity of a substance. It provides all of the equation
From playlist New AP & General Chemistry Video Playlist
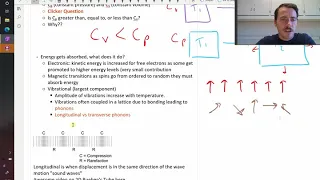
An important thermal property is heat capacity which is the amount of energy that gets absorbed to make some quantity of material increase 1 degree in temperature. This can be per mole or per mass (specific heat capacity). Heat capacity increases until the Dulong-Petit limit of 3R above th
From playlist Materials Sciences 101 - Introduction to Materials Science & Engineering 2020

Specific Heat Capacity | Matter | Physics | FuseSchool
Specific Heat Capacity | Matter | Physics | FuseSchool You might have noticed that if you are trying to boil a lot of water it takes longer than if you only wish to boil a small amount of water. This is all because of something called ‘heat capacity’. Keep watching to learn more. Befo
From playlist PHYSICS

Physics - Thermodynamics: (3 of 22) Molar Heat Capacity Of A Gas
Visit http://ilectureonline.com for more math and science lectures! In this video I will explain the molar heat capacity of a gas.
From playlist PHYSICS - THERMODYNAMICS

Physics - Thermodynamics: (6 of 14) Efficiency of a Heat Engine: Example 2
Visit http://ilectureonline.com for more math and science lectures! In this video I will show you how to calculate the efficiency of an (heat) engine, example 2.
From playlist PHYSICS - THERMODYNAMICS
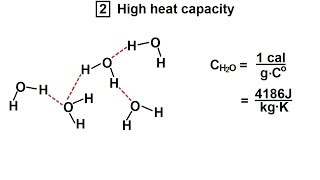
Chemistry - Liquids and Solids (16 of 59) Structures & Properties of H2O: High Heat Capacity
Visit http://ilectureonline.com for more math and science lectures! In this video I will explain the structures and properties of water (high heat capacity).
From playlist CHEMISTRY 16 LIQUIDS AND SOLIDS

Specific Heat Capacity Problems & Calculations - Chemistry Tutorial - Calorimetry
This chemistry video tutorial explains the concept of specific heat capacity and it shows you how to use the formula to solve specific heat capacity problems. This video contains plenty of examples, notes, and practice problems with the calculations to help you master this topic. My E-B
From playlist New Physics Video Playlist
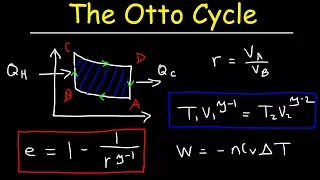
Otto Cycle of Internal Combustion Engines, Gamma vs Compression Ratio, Adiabatic Processes - Physics
This physics video tutorial provides a basic introduction into the otto cycle of an internal combustion engine. The first step is an adiabatic process when the gas undergoes compression. As the volume decreases, the temperature of the gas increases and the pressure greatly increases favo
From playlist New Physics Video Playlist

Internal Energy of an Ideal Gas - Molar Heat Capacity of Monatomic & Diatomic Gases, Gamma Ratio,
This physics video tutorial explains how to calculate the internal energy of an ideal gas - this includes monatomic gases and diatomic gases. You need to know how to calculate the molar heat capacity of a gas especially if you're given the gamma ratio of a gas. This thermodynamics video
From playlist New Physics Video Playlist

Thermodynamics 1b - Heat and Temperature II
Here we complete our discussion of the concepts of heat and temperature.
From playlist Thermodynamics
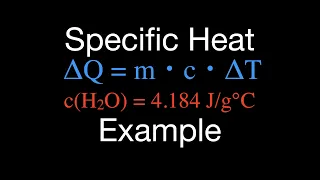
Thermodynamics: Specific Heat Capacity Calculations
This video explains how to calculate the change in heat, the change in temperature and the specific heat of a substance. The change in heat is calculated as the mass of the substance times the specific heat of the substance times the change in temperature of the substance. Q = m x c x T
From playlist Thermal Physics/Fluid Mechanics

Field Induced Quantum Phase Transitions in one-Dimensional Spin...by Ramesh Chandra Nath
PROGRAM FRUSTRATED METALS AND INSULATORS (HYBRID) ORGANIZERS Federico Becca (University of Trieste, Italy), Subhro Bhattacharjee (ICTS-TIFR, India), Yasir Iqbal (IIT Madras, India), Bella Lake (Helmholtz-Zentrum Berlin für Materialien und Energie, Germany), Yogesh Singh (IISER Mohali, In
From playlist FRUSTRATED METALS AND INSULATORS (HYBRID, 2022)

Carnot Heat Engines, Efficiency, Refrigerators, Pumps, Entropy, Thermodynamics - Second Law, Physics
This physics tutorial video shows you how to solve problems associated with heat engines, carnot engines, efficiency, work, heat, refrigerators, heat pumps and entropy. It discusses the concepts of the first and second law of thermodynamics. This video contains plenty of examples and pra
From playlist New Physics Video Playlist

Thermodynamics and Chemical Dynamics 131C. Lecture 13. The Carnot Cycle.
UCI Chem 131C Thermodynamics and Chemical Dynamics (Spring 2012) Lec 13. Thermodynamics and Chemical Dynamics -- The Carnot Cycle -- View the complete course: http://ocw.uci.edu/courses/chem_131c_thermodynamics_and_chemical_dynamics.html Instructor: Reginald Penner, Ph.D. License: Creati
From playlist Chemistry 131C: Thermodynamics and Chemical Dynamics
Mod-01 Lec-11 Thermal Conductivity of Metals
Condensed Matter Physics by Prof. G. Rangarajan, Department of Physics, IIT Madras. For more details on NPTEL visit http://nptel.iitm.ac.in
From playlist NPTEL: Condensed Matter Physics - CosmoLearning.com Physics Course

Shannon 100 - 28/10/2016 - Ruediger URBANK
Happy Numbers: 68 Years of Coding, 6² + 8² = 100 Years of Shannon, 1² + 0² + 0² = 1 Goal Ruediger Urbank (EPFL) This year, we celebrate Shannon’s 100th birthday and it has been 68 years since he laid the foundations of communications. To realize his number 1 goal or error free communica
From playlist Shannon 100

Preliminary Chemistry Water Specific heat capacity
From playlist Preliminary Chemistry - Water
