
Physics - Nuclear Physics (1 of 22) Mass of Proton, Neutron, and Electron
Visit http://ilectureonline.com for more math and science lectures! In this video I will show you how to find the mass of proton, neutron, and electron.
From playlist MOST POPULAR VIDEOS
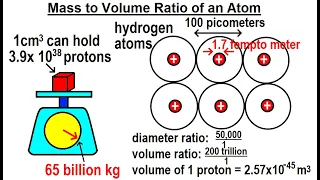
Can You Believe It? #20 What is the Mass to Volume Ratio of an Atom?
Visit http://ilectureonline.com for more math and science lectures! To donate: http://www.ilectureonline.com/donate https://www.patreon.com/user?u=3236071 We will learn the mass to volume ratio of an atom of the proton mass and how many protons can an 1 cm^3 hold. Previous video in this
From playlist CAN YOU BELIEVE IT?

Teach Astronomy - Atomic Structure
http://www.teachastronomy.com/ Atoms are tiny pieces of matter, but they are composed of even smaller particles. Orbiting the nucleus of every atom are electrons. Electrons have negative charge and a mass of only nine times ten to the minus thirty-one kilograms. There are 1,836 times li
From playlist 04. Chemistry and Physics

Physics - Ch 66.5 Quantum Mechanics: The Hydrogen Atom (41 of 78) What is the Reduced Mass?
Visit http://ilectureonline.com for more math and science lectures! In this video I will explain what is “the reduced mass” of an electron. Reduced mass is the effective mass. Because of the interaction when the electron revolves around the nucleus, the nucleus the does not stay in the ce
From playlist THE "WHAT IS" PLAYLIST

Atomic mass unit explained and its relationship to the kilogram
The atomic mass unit is a non-SI unit for mass regualrly used in nuclear physics. since it allows for easier comparisons between atoms. I discuss how it came about, how it relates to the kilogram and the eV/c2, and how to use it in nuclear reactions See www.physicshigh.com for all my vi
From playlist Modern Physics

Physics - Nuclear Physics (5 of 22) Volume of Earth as a Nuclear Ball
Visit http://ilectureonline.com for more math and science lectures! In this video I will show you how to find the volume of our Earth as a nuclear ball.
From playlist MODERN PHYSICS 2: ATOMIC AND NUCLEAR PHYSICS, PARTICLE PHYSICS

Atomic number, atomic mass, protons, neutrons and electrons: from fizzics.org
Atomic number and atomic mass together provide key information about an isotope of an element. The two numbers prefix the symbol, the one above giving the atomic mass and the one below the atomic number. The atomic number or proton number defines which element it is. By subtracting that fr
From playlist Atomic structure

Watch more videos on http://www.brightstorm.com/science/chemistry SUBSCRIBE FOR All OUR VIDEOS! https://www.youtube.com/subscription_center?add_user=brightstorm2 VISIT BRIGHTSTORM.com FOR TONS OF VIDEO TUTORIALS AND OTHER FEATURES! http://www.brightstorm.com/ LET'S CONNECT! Facebook ► h
From playlist Chemistry

Physics - Ch 66.5 Quantum Mechanics: The Hydrogen Atom (26 of 78) Orbital Quantum Number vid 2
Visit http://ilectureonline.com for more math and science lectures! In this video I will further explain the orbital quantum number l representing the angular momentum (magnitude and direction) of the electron. Next video in this series can be seen at: https://youtu.be/E7BM951tNNA
From playlist PHYSICS 66.5 QUANTUM MECHANICS: THE HYDROGEN ATOM

Lecture 9 | New Revolutions in Particle Physics: Standard Model
(March 30, 2009) Leonard Susskind explains how the Higgs phenomenon interacts masses of quarks and leptons. This course is a continuation of the Fall quarter on particle physics. The material will focus on the Standard Model of particle physics, especially quantum chromodynamics (the the
From playlist Lecture Collection | Particle Physics: Standard Model

Mass Defect & Binding Energy (2 of 7), The Nucleus
Goes over the required values of mass and energy for the neutron, proton, electron and hydrogen atom. Values are given in kilograms (kg), unified atomic mass units (u) and mega electron volts (MeV). The mass of an atomic nucleus is less than the sum of the individual masses of the free co
From playlist Mass Defect and Binding Energy

What is the Chandrasekhar limit for White Dwarf Stars?
This video provides a simplified step by step derivation of the Chandrasekhar limit for White Dwarf stars. After briefly discussing the history of white dwarf stars, an overview of electron degeneracy pressure is provided. Using a combination of quantum mechanics and Einstein's theory of s
From playlist Relativity

Kronig-Penny Model: Effective Mass
Here I go cover energy/momentum diagrams in more depth, and I explain how we can use them to determine how electrons and holes move in the semiconductor through the concept of effective mass. https://www.patreon.com/edmundsj If you want to see more of these videos, or would like to say th
From playlist Electronics I: Semiconductor Physics and Devices

A2 Physics Exam Questions: Quantum, Nuclear, Particles
Examples of exam questions at Physics A2 level for Quantum, Nuclear, Particles covering Edexcel, AQA and OCR material.
From playlist A Level Physics Exam Questions - Worked Examples

UCI Physics 3C: Basic Physics III (Fall 2013) Lec 21. Basic Physics III View the complete course: http://ocw.uci.edu/courses/physics_3c_basic_physics_iii.html Instructor: Michael Smy, Ph.D. License: Creative Commons CC-BY-SA Terms of Use: http://ocw.uci.edu/info More courses at http://ocw
From playlist Physics 3C: Basic Physics III

Radioactivity (5 of 16) Nuclear Fusion, An Explanation
Explains nuclear fusion and the resulting mass defect and binding energy. An example problem is done for the nuclear fusion of two deuterium atoms into tritium and one hydrogen atom. Social Media for Step by Step Science: Teacher Pay Teachers Store: https://tinyurl.com/y6d2cdfj Instagra
From playlist Radioactivity

This chemistry video tutorial provides a basic introduction into atoms. Atoms are made up of subatomic particles called protons, neutrons, and electrons. This video discusses the structure of atoms as well as the atomic number, mass number, and average atomic mass of the elements. My We
From playlist New AP & General Chemistry Video Playlist
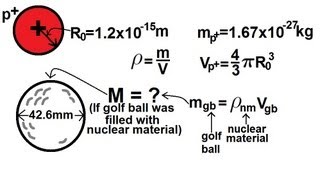
Physics - Nuclear Physics (4 of 22) Density of a Nucleus
Visit http://ilectureonline.com for more math and science lectures! In this video I will show you how to find the density of a nucleus.
From playlist MODERN PHYSICS 2: ATOMIC AND NUCLEAR PHYSICS, PARTICLE PHYSICS

Neutrino Physics III - André de Gouvêa
Prospects in Theoretical Physics Particle Physics at the LHC and Beyond Topic: Neutrino Physics III Speaker: André de Gouvêa Date: July 21th, 2017
From playlist PiTP 2017