Chirality (chemistry)
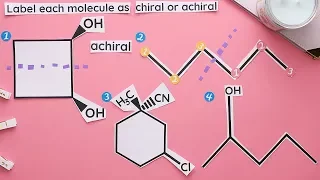
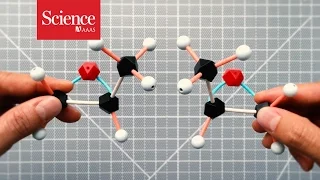
In chemistry, a molecule or ion is called chiral (/ˈkaɪrəl/) if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality (/kaɪˈrælɪti/). The terms are derived from Ancient Greek χείρ (cheir) 'hand'; which is the canonical example of an object with this property. A chiral molecule or ion exists in two stereoisomers that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physical properties, except that they often have opposite optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic, and it usually differs chemically and physically from the pure enantiomers. Chiral molecules will usually have a stereogenic element from which chirality arises. The most common type of stereogenic element is a stereogenic center, or stereocenter. In the case of organic compounds, stereocenters most frequently take the form of a carbon atom with four distinct groups attached to it in a tetrahedral geometry. A given stereocenter has two possible configurations, which give rise to stereoisomers (diastereomers and enantiomers) in molecules with one or more stereocenter. For a chiral molecule with one or more stereocenter, the enantiomer corresponds to the stereoisomer in which every stereocenter has the opposite configuration. An organic compound with only one stereogenic carbon is always chiral. On the other hand, an organic compound with multiple stereogenic carbons is typically, but not always, chiral. In particular, if the stereocenters are configured in such a way that the molecule has an internal plane of symmetry, then the molecule is achiral and is known as a meso compound. Less commonly, other atoms like N, P, S, and Si can also serve as stereocenters, provided they have four distinct substituents (including lone pair electrons) attached to them. Molecules with chirality arising from one or more stereocenters are classified as possessing central chirality. There are two other types of stereogenic elements that can give rise to chirality, a stereogenic axis (axial chirality) and a stereogenic plane (planar chirality). Finally, the inherent curvature of a molecule can also give rise to chirality (inherent chirality). These types of chirality are far less common than central chirality. BINOL is a typical example of an axially chiral molecule, while trans-cyclooctene is a commonly cited example of a planar chiral molecule. Finally, helicene possesses helical chirality, which is one type of inherent chirality. Chirality is an important concept for stereochemistry and biochemistry. Most substances relevant to biology are chiral, such as carbohydrates (sugars, starch, and cellulose), the amino acids that are the building blocks of proteins, and the nucleic acids. In living organisms, one typically finds only one of the two enantiomers of a chiral compound. For that reason, organisms that consume a chiral compound usually can metabolize only one of its enantiomers. For the same reason, the two enantiomers of a chiral pharmaceutical usually have vastly different potencies or effects. (Wikipedia).