
Physics - Thermodynamics: (3 of 22) Molar Heat Capacity Of A Gas
Visit http://ilectureonline.com for more math and science lectures! In this video I will explain the molar heat capacity of a gas.
From playlist PHYSICS - THERMODYNAMICS

From playlist h. Three-Dimensional Measurement
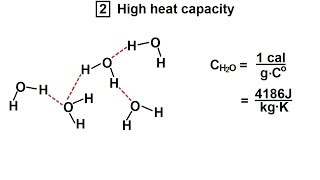
Chemistry - Liquids and Solids (16 of 59) Structures & Properties of H2O: High Heat Capacity
Visit http://ilectureonline.com for more math and science lectures! In this video I will explain the structures and properties of water (high heat capacity).
From playlist CHEMISTRY 16 LIQUIDS AND SOLIDS

What Is The Difference Between Specific Heat Capacity, Heat Capacity, and Molar Heat Capacity
This chemistry video tutorial explains the difference between specific heat capacity, heat capacity, and molar heat capacity. It contains a few examples and practice problems on calculating the specific heat capacity and molar heat capacity of a substance. It provides all of the equation
From playlist New AP & General Chemistry Video Playlist
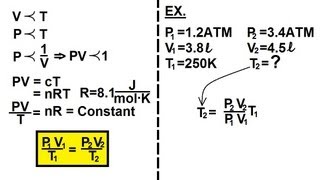
Physics - Thermodynamics: States: (5 of 10) Ideal Gas Equation
Visit http://ilectureonline.com for more math and science lectures! In this video I will explain and show you how to find temperature using the ideal gas equation. Next video in this series can be seen at: https://youtu.be/SUzaH162LY4
From playlist PHYSICS - THERMODYNAMICS
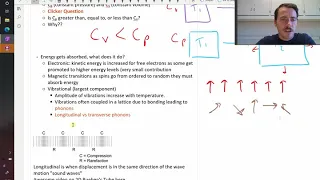
An important thermal property is heat capacity which is the amount of energy that gets absorbed to make some quantity of material increase 1 degree in temperature. This can be per mole or per mass (specific heat capacity). Heat capacity increases until the Dulong-Petit limit of 3R above th
From playlist Materials Sciences 101 - Introduction to Materials Science & Engineering 2020

Solid Hydrogen Explained (Again) - Is it the Future of Energy Storage?
Corrections: I've trimmed out a couple of sections from this video that misstated some facts about Plasma Kinetics and hydrogen production. I apologize for any confusion around this. As I mention in the video, I'm learning and trying to make each video better than the last. Thanks for t
From playlist Explained
Thermal properties of materials
0:00 introduction 1:35 heat capacity 4:31 different types of atomic vibrations (phonons), longitudinal (acoustic) vs transverse (optical) 8:31 minimum phonon wavelength 12:23 temperature dependence of heat capacity (Debye temperature, Dulong Petit limit) 14:38 thermal expansion 17:39 typic
From playlist Introduction to Materials Science and Engineering Fall 2018
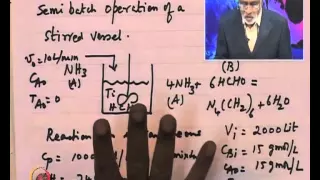
Mod-01 Lec-17 Illustrative Example : Energy Balance in Stirred Vessels
Advanced Chemical Reaction Engineering (PG) by Prof. H.S.Shankar,Department of Chemical Engineering,IIT Bombay.For more details on NPTEL visit http://nptel.ac.in
From playlist IIT Bombay: Advanced Chemical Reaction Engineering | CosmoLearning.org

How Much Work Does GAS Do When It EXPANDS?!? #Physics #Chemistry #Thermodynamics #College #NicholasGKK #Shorts
From playlist Heat and Chemistry

Chemistry of Gases (13 of 40) Density of Gases: Other Forms
Visit http://ilectureonline.com for more math and science lectures! In this video I will show you how to find the density (in forms of n/V or N/V) of a gas using the ideal gas equation.
From playlist CHEMISTRY 10 THE CHEMISTRY OF GASES
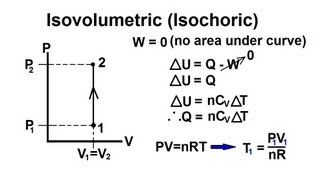
Physics - Thermodynamics: (9 of 22) Change Of State: Constant Volume (Isovolumetric)
Visit http://ilectureonline.com for more math and science lectures! In this video I will explain the change of state of a constant volume or isovolumetric (isochoric) process.
From playlist PHYSICS - THERMODYNAMICS

0:00 heat capacity 3:45 phonons as a means to store heat (transverse vs longitudinal phonons) 9:30 thermal expansion 14:00 thermal conductivity 23:25 thermal stresses 25:42 thermal shock resistance
From playlist Introduction to Materials Science & Engineering Fall 2019

The Atmosphere, the Ocean and Environmental Change (GG 140) The material covered throughout the course is reviewed. Properties of air and water are discussed. Hydrostatic balance is discussed as related to the atmosphere, ocean and solid earth. Geostrophic balance is a force balance
From playlist Atmosphere, Ocean and Environmental Change with Ron Smith

Volume and Capacity (Converting between units of volume)
More resources available at www.misterwootube.com
From playlist Applications of Measurement
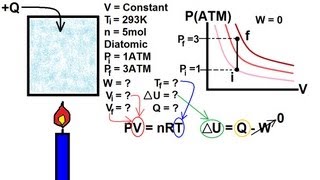
Physics - Thermodynamics:(10 of 22):Change of State: Constant Volume (Isovolumetric)
Visit http://ilectureonline.com for more math and science lectures! In this video I will show you how to calculate the change of the internal energy of a gas of an isovolumetric process.
From playlist PHYSICS - THERMODYNAMICS
Stanford Webinar - The Next Big Opportunities in Energy Storage, William Chueh
https://online.stanford.edu/energy Global energy storage on the grid is expected to double what it is today by 2021. Countries such as Japan, India, Germany, the United Kingdom and the United States are preparing to take advantage of this shift through research, policy and integration. Thi
From playlist Environment & Energy

MIT 3.054 Cellular Solids: Structure, Properties and Applications, Spring 2015 View the complete course: http://ocw.mit.edu/3-054S15 Instructor: Lorna Gibson Thermal properties and behaviors of foams is covered in this session. License: Creative Commons BY-NC-SA More information at http:
From playlist MIT 3.054 Cellular Solids: Structure, Properties and Applications, Spring 2015

Why 3D Printing Batteries Matters
3D Printing Solid State Batteries? Explained. For a limited time, new customers can try Amazon Music Unlimited FREE for three months! No credit card required! Go to https://amazon.com/undecided. Renews automatically, cancel anytime. Terms apply. 3D printing has become popular with manufact
From playlist Battery technology
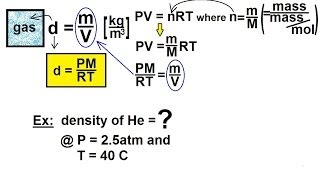
Chemistry of Gases (12 of 40) Density of Gases: Basic
Visit http://ilectureonline.com for more math and science lectures! In this video I will show you how to find the density of a gas using the ideal gas equation.
From playlist CHEMISTRY 10 THE CHEMISTRY OF GASES