VSEPR theory
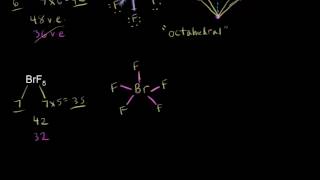
Valence shell electron pair repulsion (VSEPR) theory (/ˈvɛspər, vəˈsɛpər/ VESP-ər, və-SEP-ər), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm. The premise of VSEPR is that the valence electron pairs surrounding an atom tend to repel each other and will, therefore, adopt an arrangement that minimizes this repulsion. This in turn decreases the molecule's energy and increases its stability, which determines the molecular geometry. Gillespie has emphasized that the electron-electron repulsion due to the Pauli exclusion principle is more important in determining molecular geometry than the electrostatic repulsion. The insights of VSEPR theory are derived from topological analysis of the electron density of molecules. Such quantum chemical topology (QCT) methods include the electron localization function (ELF) and the quantum theory of atoms in molecules (AIM or QTAIM). Hence, VSEPR is unrelated to wave function-based methods such as orbital hybridisation in valence bond theory. (Wikipedia).