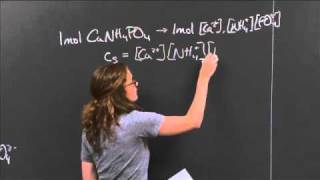Ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula NH+4 or [NH4]+. It is formed by the protonation of ammonia (NH3). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations ([NR4]+), where one or more hydrogen atoms are replaced by organic groups (indicated by R). (Wikipedia).